



-
Description
-
Shipping Policy
-
.ic_80_wrap {margin:0 auto;width:100%;max-width:1000px;box-sizing:border-box;} .ic_80 {margin:0 auto; width:100%; max-width:1000px; line-height:0;padding:0;text-align:center;} .ic_80 img {margin:0 auto; width:100%; max-width:1000px;}




























.mrsc_wrap {margin:0 auto;width:100%;max-width:1000px;box-sizing:border-box;} .mrsc {margin:0 auto; width:100%; max-width:1000px; line-height:0;padding:0;text-align:center;} .mrsc img {margin:0 auto; width:100%; max-width:1000px;} .gifbg01 {background-color:#fff; } .gifbg01 img {max-width:860px;}
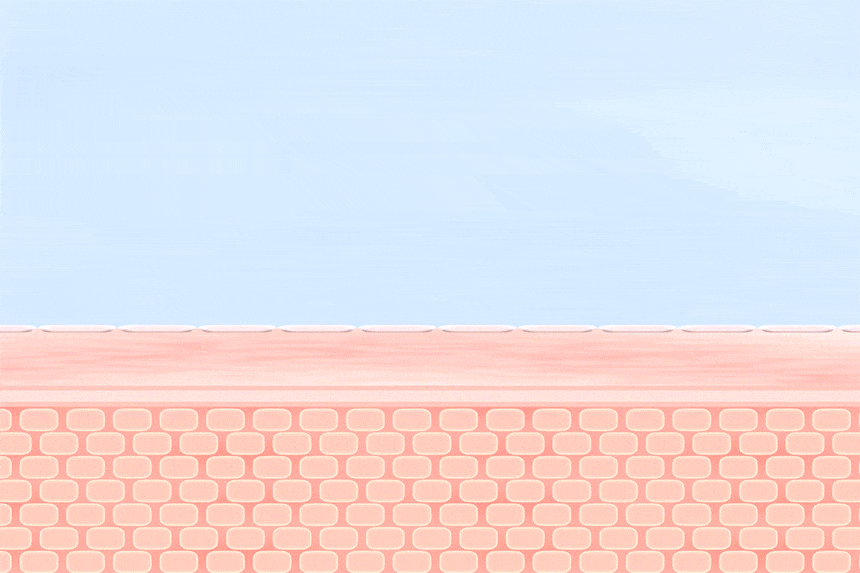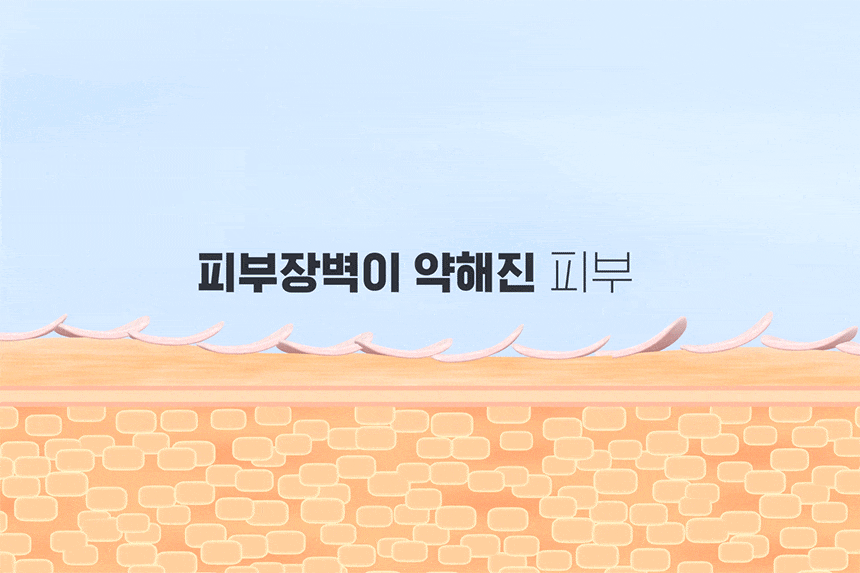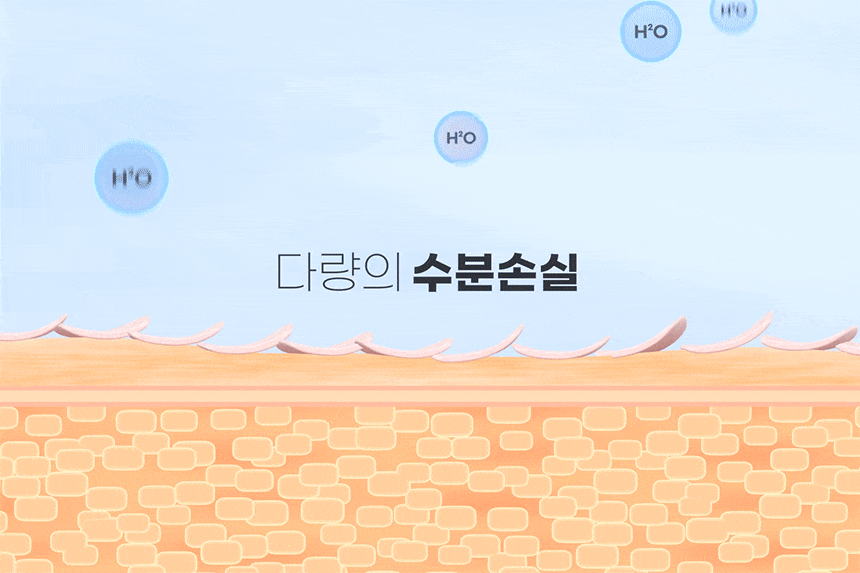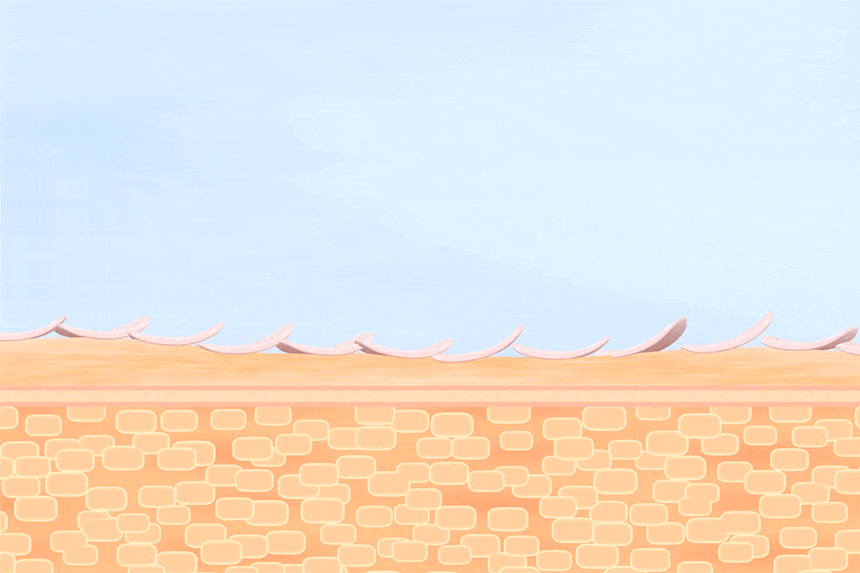
Product Information Provision Notice Table Dose or weight of the contents 50ml Main specifications For all skin types Usage date (or period of use after opening) 36 months from the date of manufacture before opening, and within 12 months after opening How to use Take an appropriate amount of the contents and apply evenly to skin exposed to UV rays. (TIP: When washing your face after using sunscreen, it is recommended to use a special cleanser to clean it thoroughly.) Cosmetics manufacturers, cosmetics responsible vendors and customized cosmetics vendors Kolmar Korea/Neopharm Manufacture Republic of Korea All ingredients that must be listed in accordance with the Cosmetics Act Water, dibutyl adipate, propanediol, glycerin, diethylaminohydroxybenzoyl hexylbenzoate, titanium dioxide, ethylhexyltriazone, caprylyl methicone, caprylic/capric triglyceride, polyglyceryl-3 distearate, diethylhexyl butamidottriasone, 1,2-Hexanediol, Pentylene Glycol, Sodium Hyaluronate, Glyceryl Stearate, PolyC10-30 Alkyl Acrylate, Dimethicone/Vinyl Dimethicone Crosspolymer, Polyhydroxystearic Acid, Dimethiconol, Trisiloxane, Methyl Propanediol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Alumina, Stearic Acid, Glyceryl Stearate Citrate, Polyacrylate Crosspolymer-6, Ethylhexylglycerin, Xanthan Gum, Sodium Polyacrylate, Polyether-1, t-Butyl Alcohol, Myristoyl/Palmitoyl Oxosteamide/Aracamide MEA, Biosaccharide Gum-1, Panthenol, Tocopherol, Madecassoside, Sodium Acetylated Hyaluronate, Sodium Hyaluronate Crosspolymer, Potassium hyaluronate, hydroxypropyltrimonium hyaluronate, hydrolyzed hyaluronic acid, hyaluronic acid Functional cosmetics food and medicine safety department examination Functional cosmetics examination (or report) in accordance with the Cosmetics Act Precautions when using 1. If you have any abnormal symptoms or side effects such as red spots, swelling, or RYO swelling in the area of use due to direct sunlight when using cosmetics or after use, consult a specialist 2. Refrain from using it on areas with wounds 3. Precautions for storage and handling A) Store out of reach of children B) Store away from direct sunlight Quality Assurance Standards In accordance with relevant laws and consumer dispute resolution regulations Consumer Counseling Phone Number 080-500-0037